Module 5: Personalized medicine
The Human Genome Project and the Human Microbiome Project have both produced information and technology that can then be utilized by the healthcare field to improve patient care on a day-to-day basis as well as in disease management.
What is personalized medicine?
In the advent of the completion of the Human Genome Project, personalized medicine is becoming more of a reality. Personalized medicine is a branch of healthcare that utilizes a patient's unique clinical, genomic, historical, and environmental information to inform both the treatment of disease in that individual but also to maintain a state of wellness. The goal of personalized medicine is to optimize healthcare for each individual rather than the average person.
In the advent of the completion of the Human Genome Project, personalized medicine is becoming more of a reality. Personalized medicine is a branch of healthcare that utilizes a patient's unique clinical, genomic, historical, and environmental information to inform both the treatment of disease in that individual but also to maintain a state of wellness. The goal of personalized medicine is to optimize healthcare for each individual rather than the average person.
How can personalized medicine be used to predict a person's risk for a disease?
There are already many tools available to better personalize the risk of an individual developing a certain disease. One of these is a simple survey of an individual's family health history. The family health history takes into account shared genetic and environmental factors that influence a person's health. The risk of a person developing diseases such as type 2 diabetes, coronary artery disease, colon cancer, lung cancer, and breast cancer (all of which are among the top killers of Americans) is increased by having a relative with that disease. An assessment of a person's family health history allows doctors to identify people at higher risk for a disease and then recommend early interventions as well as early screening to either prevent the development of the disease or to at least catch it in an early stage.
You may of heard of people with certain mutations in the BRCA1 and BRCA2 genes who are at a significantly higher risk for the development of breast cancer. People who carry these mutations can then make the decision to take preventative measures to minimize their risk or to visit their doctor more frequently for screening in hopes of detecting breast cancer in an early, more treatable stage. Scientists are currently looking for mutations in other genes that may increase a person's risk for developing other diseases. Once these mutations are identified they could be potentially be routinely screened for in all individuals. Identification and screening for these high risk mutations in people will allow for early intervention to either prevent or delay the development and progression of disease.
However, while this plan is seemingly a fantastic way to identify people who are at high risk for a certain disease, unfortunately it is very difficult for scientists to find genetic markers for increased disease risk that are as useful as the mutations in
BRCA1 and BRCA2. This is because these risk markers need to be wide-spread enough and increase a person's risk significantly enough to be useful in a healthcare setting. If a risk marker was incredibly rare, very few people would have it and so screening everybody for that marker would be useless. If the risk marker did not significantly increase a person's risk for developing a disease, then having the marker would not indicate cause for potentially invasive prevention strategies or having to come into your doctor more often for screening.
There are already many tools available to better personalize the risk of an individual developing a certain disease. One of these is a simple survey of an individual's family health history. The family health history takes into account shared genetic and environmental factors that influence a person's health. The risk of a person developing diseases such as type 2 diabetes, coronary artery disease, colon cancer, lung cancer, and breast cancer (all of which are among the top killers of Americans) is increased by having a relative with that disease. An assessment of a person's family health history allows doctors to identify people at higher risk for a disease and then recommend early interventions as well as early screening to either prevent the development of the disease or to at least catch it in an early stage.
You may of heard of people with certain mutations in the BRCA1 and BRCA2 genes who are at a significantly higher risk for the development of breast cancer. People who carry these mutations can then make the decision to take preventative measures to minimize their risk or to visit their doctor more frequently for screening in hopes of detecting breast cancer in an early, more treatable stage. Scientists are currently looking for mutations in other genes that may increase a person's risk for developing other diseases. Once these mutations are identified they could be potentially be routinely screened for in all individuals. Identification and screening for these high risk mutations in people will allow for early intervention to either prevent or delay the development and progression of disease.
However, while this plan is seemingly a fantastic way to identify people who are at high risk for a certain disease, unfortunately it is very difficult for scientists to find genetic markers for increased disease risk that are as useful as the mutations in
BRCA1 and BRCA2. This is because these risk markers need to be wide-spread enough and increase a person's risk significantly enough to be useful in a healthcare setting. If a risk marker was incredibly rare, very few people would have it and so screening everybody for that marker would be useless. If the risk marker did not significantly increase a person's risk for developing a disease, then having the marker would not indicate cause for potentially invasive prevention strategies or having to come into your doctor more often for screening.
How can personalized medicine be used to improve disease diagnosis?
While different people may be diagnosed with the same disease, many complex diseases can often be broken down into clinical subtypes. A person's disease subtype may effect their symptoms, their disease progression, and their response to different medications. As such, accurate identification of a person's particular disease subtype is crucial to personalizing their treatment plan.
For example, when the genome of the tumors of multiple people with the same cancer diagnosis are sequenced, genetic differences among the tumors are observed. These differences are distinct enough to allow the cancer to be sorted into different subtypes.Personalized disease subtyping can easily be applied to other conditions as well by looking at different patterns of gene expression, protein production, and chemical production by cells in the human body.
While different people may be diagnosed with the same disease, many complex diseases can often be broken down into clinical subtypes. A person's disease subtype may effect their symptoms, their disease progression, and their response to different medications. As such, accurate identification of a person's particular disease subtype is crucial to personalizing their treatment plan.
For example, when the genome of the tumors of multiple people with the same cancer diagnosis are sequenced, genetic differences among the tumors are observed. These differences are distinct enough to allow the cancer to be sorted into different subtypes.Personalized disease subtyping can easily be applied to other conditions as well by looking at different patterns of gene expression, protein production, and chemical production by cells in the human body.
How can personalized medicine be used to determine an appropriate treatment for a person?
Pharmacogenomics is a subset of personalized medicine that involves using a person's genomic information to predict their response to a disease. In the treatment of acute lymphoblastic leukemia and breast cancers, patients whose tumor cells express certain genes at a high level are resistant to traditional treatment methods.
Another example is the determination of the proper wafarin dosage to prevent blood clots. Slight variations in two genes among individuals -- known as polymorphisms-- account for many of the different responses to the drug seen in people. The fact that an individual's drug response can be predicted from a person's genome ahead of time allows for doctors to set a more appropriate drug dosage from the beginning and potentially prevent the negative side effects of an incorrect dosage.
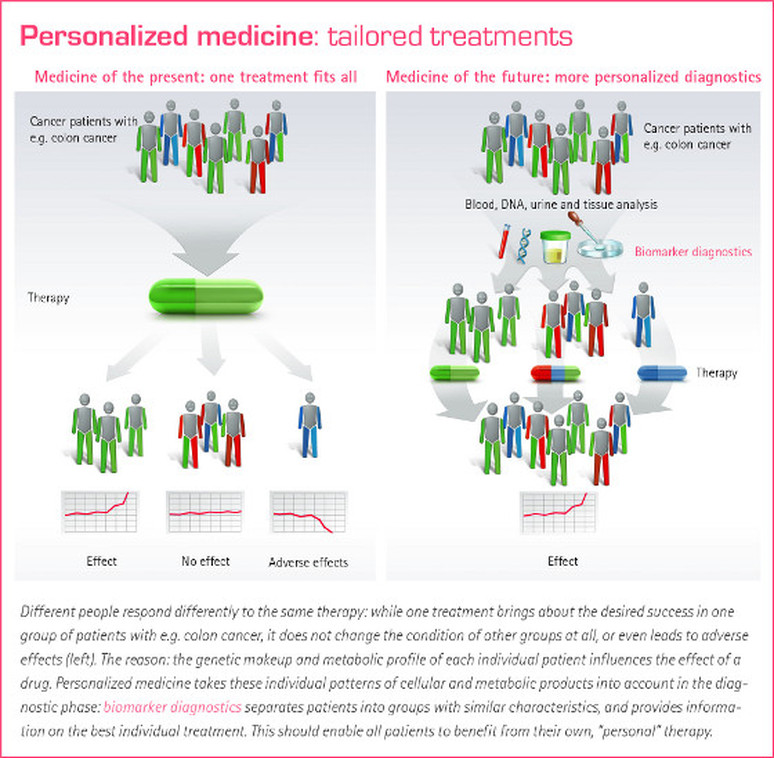
Drugs on the market right now are useful for the average person with a particular medical condition. Unfortunately, not everybody with a disease closely resembles the average due to genetic and environmental differences among people. In many people the drug may be effective. But in others it may have no effect at all or even adverse effects (Figure 1). As we move closer to a future of personalized medicine, patient's will be tested for genetic and physical differences that may impact the efficacy of a particular drug treatment. They will then be administered a medication that is appropriate for them, not the average (Figure 1).
Pharmacogenomics is a subset of personalized medicine that involves using a person's genomic information to predict their response to a disease. In the treatment of acute lymphoblastic leukemia and breast cancers, patients whose tumor cells express certain genes at a high level are resistant to traditional treatment methods.
Another example is the determination of the proper wafarin dosage to prevent blood clots. Slight variations in two genes among individuals -- known as polymorphisms-- account for many of the different responses to the drug seen in people. The fact that an individual's drug response can be predicted from a person's genome ahead of time allows for doctors to set a more appropriate drug dosage from the beginning and potentially prevent the negative side effects of an incorrect dosage.
Drugs on the market right now are useful for the average person with a particular medical condition. Unfortunately, not everybody with a disease closely resembles the average due to genetic and environmental differences among people. In many people the drug may be effective. But in others it may have no effect at all or even adverse effects (Figure 1). As we move closer to a future of personalized medicine, patient's will be tested for genetic and physical differences that may impact the efficacy of a particular drug treatment. They will then be administered a medication that is appropriate for them, not the average (Figure 1).
Can a person's microbiome help to improve their healthcare?
As mentioned in "What your microbes do for you", "The microbiome and disease", and "The microbiome and drug metabolism", the bacterial communities within you play a major role in your body's day-to-day functioning (or malfunctioning, in some cases). As the sequencing of an individual's microbiome becomes easier and more affordable, the information that we already know about the interactions between the microbiome and drug metabolism as well as the microbiome's role in disease development can help doctors personalize healthcare to not to an individual but also their microbes.
As mentioned in "What your microbes do for you", "The microbiome and disease", and "The microbiome and drug metabolism", the bacterial communities within you play a major role in your body's day-to-day functioning (or malfunctioning, in some cases). As the sequencing of an individual's microbiome becomes easier and more affordable, the information that we already know about the interactions between the microbiome and drug metabolism as well as the microbiome's role in disease development can help doctors personalize healthcare to not to an individual but also their microbes.
Module 5 Questions:
1.How does personalized medicine differ from medical practice currently?
2.How can a person’s microbiome information be used to personalize their healthcare?
1.How does personalized medicine differ from medical practice currently?
2.How can a person’s microbiome information be used to personalize their healthcare?